创新的癌症治疗使用超声激活药物靶向
诸平
据美国加州理工学院(California Institute Of Technology)2024年3月2日提供的消息,该校研究人员的创新癌症治疗使用超声激活药物进行靶向治疗(Innovative Cancer Treatment Uses Ultrasound-Activated Drugs for Targeting), 减少了副作用,提高了疗效。
治疗癌症的化学疗法(Chemotherapy)是20世纪最成功的医学成果之一,但它还远远不够完美。任何经历过化疗的人,或者有朋友或爱人经历过化疗的人,都会熟悉它的许多副作用:脱发、恶心、免疫系统减弱,甚至不孕和神经损伤。
这是因为化疗药物是有毒的。它们的目的是通过毒害癌细胞来杀死癌细胞,但由于癌细胞来自健康细胞,并且与健康细胞本质上相似,因此很难制造出一种既杀死癌细胞又不损害健康组织的药物。
靶向给药的突破(Breakthrough in Targeted Drug-Delivery)
但现在,加州理工学院的两个研究团队创造了一种全新的药物输送系统,他们说,这种系统最终可能会让医生有能力以更有针对性的方式治疗癌症。该系统使用的药物由超声波激活,只在体内需要的地方使用。
该系统是化学助理教授麦克斯韦·罗伯(Maxwell Robb)和马克斯·德尔布吕克化学工程与医学工程教授(Max Delbrück Professor of Chemical Engineering and Medical Engineering)兼霍华德·休斯医学研究所(Howard Hughes Medical Institute)研究员米哈伊尔·夏皮罗(Mikhail Shapiro)合作在实验室开发的。相关研究结果于2023年9月19日已经在《美国国家科学院院刊》(Proceedings of the National Academy of Sciences)网站发表——Yuxing Yao, Molly E. McFadden, Stella M. Luo, Ross W. Barber, Elin Kang, Avinoam Bar-Zion, Cameron A. B. Smith, Zhiyang Jin, Mark Legendre, Bill Ling, Dina Malounda, Andrea Torres, Tiba Hamza, Chelsea E. R. Edwards, Mikhail G. Shapiro, Maxwell J. Robb. Remote control of mechanochemical reactions under physiological conditions using biocompatible focused ultrasound. Proceedings of the National Academy of Sciences, 2023, 120 (39): e2309822120. DOI: 10.1073/pnas.2309822120. September 19, 2023. https://doi.org/10.1073/pnas.2309822120
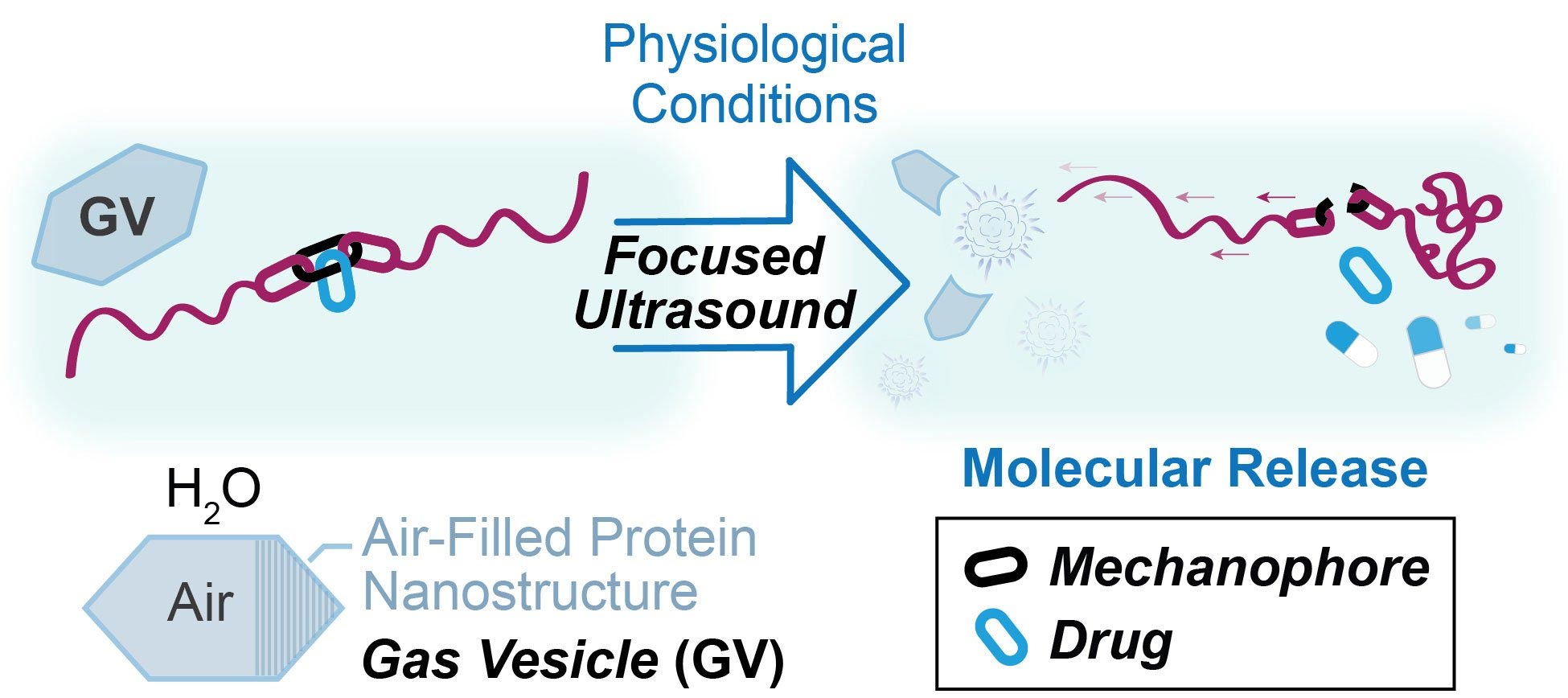
在此文中,研究人员展示了他们是如何结合各自专业的元素来创建这个平台的。两个研究小组通过合作,将气体囊泡(gas vesicles)——即在某些细菌中发现的充满空气的蛋白质胶囊——和机械应力聚合物(mechanophores)——即受到物理力时会发生化学变化的分子——结合在一起。米哈伊尔·夏皮罗的实验室以前曾将气泡与超声波结合起来,对单个细胞进行成像(image individual cells),并精确地移动细胞(move cells around)。麦克斯韦·罗伯的实验室已经创造出了在拉伸时改变颜色的机械应力聚合物,这使得它们可以用于检测结构中的应变,还有一些机械应力聚合物可以释放更小的分子,包括药物,以响应机械刺激。在这项新工作中,他们设计了一种使用超声波作为刺激的方法。
超声活化机械应力聚合物(Ultrasound-Activated Mechanophores)
麦克斯韦·罗伯说:“我们已经考虑这个问题很长时间了。当我第一次来到加州理工学院时,米哈伊尔·夏皮罗和我开始谈论超声波的机械效应。”
当他们开始研究机械应力聚合物和超声波的结合时,他们发现了一个问题:超声波可以激活机械应力聚合物,但只有在强度太大的情况下才会损伤邻近的组织。研究人员需要的是一种将超声波能量集中在他们想要的地方的方法。事实证明,米哈伊尔·夏皮罗的气体囊泡技术提供了解决方案。
在他之前的工作中,米哈伊尔·夏皮罗利用了囊泡在受到超声波轰击时振动或像铃铛一样响的倾向。然而,在目前的研究中,这些囊泡被挤得太紧以至于破裂,从而集中了超声波能量。这些囊泡有效地变成了微小的炸弹,它们的爆炸激活了机械应力聚合物。
2023届博士毕业生、上述研究报告的合著者莫莉·麦克法登(Molly McFadden)说:“通过超声波施加力通常依赖于非常强烈的条件,这种条件会触发微小的溶解气泡内爆。它们的崩溃是激活机械应力聚合物的机械力的来源。这些囊泡对超声波非常敏感。使用它们,我们发现在更弱的超声波下也可以实现相同的机械应力聚合物激活。”,
未来潜力及影响(Future Potential and Implications)
米哈伊尔·夏皮罗实验室的博士后研究员姚宇兴(Yuxing Yao音译)说,这是聚焦超声第一次能够在生物环境中控制特定的化学反应。“以前超声波被用来破坏物体或移动物体。但现在它为我们利用机械化学(mechanochemistry)开辟了一条新的道路。”
到目前为止,该平台只在受控的实验室条件下进行了测试,但在未来,研究人员计划在活体中进行测试。
这项研究得到了加州理工学院化学与化学工程系创新基金{Division of Chemistry and Chemical Engineering (CCE) at Caltech through a CCE Innovation Grant}、阿诺德和梅布尔·贝克曼基金会(Arnold and Mabel Beckman Foundation through a Beckman Young Investigator Award)、大卫和露西尔·帕卡德基金会(David and Lucille Packard Foundation)、雷斯尼克可持续发展研究所(Resnick Sustainability Institute)、协作生物技术研究所(Institute for Collaborative Biotechnologies W911NF-19-D-0001)、以及美国国立卫生研究院(NIH)的国家普通医学科学研究所(National Institute of General Medical Sciences of the NIH R35GM150988)、美国国家科学基金会(NSF)研究生研究奖学金(NSF Graduate Research Fellowship DGE-1745301)、加州理工学院的芭芭拉·布格尔奖学金(Barbara J. Burger Fellowship from Caltech)、莱斯特·多伊奇奖学金(Lester Deutsch Fellowship)、玛丽·居里博士后奖学金(Marie Skłodowska-Curie Postdoctoral Fellowship)、人类前沿科学计划跨学科奖学金(Human Frontiers Science Program Cross-Disciplinary Fellowship)、阿尔弗雷德·斯隆基金会颁发的斯隆研究奖学金(Alfred P. Sloan Foundation for a Sloan Research Fellowship)、卡米尔和亨利·德雷福斯基金会颁发的卡米尔·德雷福斯教师学者奖(Camille and Henry Dreyfus Foundation for Camille Dreyfus Teacher-Scholar Awards)的资助或支持。
https://doi.org/10.1073/pnas.2309822120
上述介绍,仅供参考。欲了解更多信息,敬请注意浏览原文或者相关报道。
Remote control of chemical reactions with spatial and temporal precision would be an enabling technology for diverse biomedical applications. Polymer mechanochemistry offers a promising approach whereby ultrasound is used to activate specific chemical transformations. However, ultrasound conditions conventionally employed in the field result in unsafe heating and tissue damage. To address this challenge, we have developed a synergistic platform that leverages air-filled protein nanostructures (gas vesicles) to serve as acousto-mechanical transducers, enabling mechanochemical activation under clinically relevant conditions using biocompatible focused ultrasound for the controlled release of small molecule payloads, including a chemotherapeutic drug. This strategy represents a promising modality for controlling chemical reactivity in biological systems and unlocks the translational potential of polymer mechanochemistry for therapeutic and bioimaging applications.
External control of chemical reactions in biological settings with spatial and temporal precision is a grand challenge for noninvasive diagnostic and therapeutic applications. While light is a conventional stimulus for remote chemical activation, its penetration is severely attenuated in tissues, which limits biological applicability. On the other hand, ultrasound is a biocompatible remote energy source that is highly penetrant and offers a wide range of functional tunability. Coupling ultrasound to the activation of specific chemical reactions under physiological conditions, however, remains a challenge. Here, we describe a synergistic platform that couples the selective mechanochemical activation of mechanophore-functionalized polymers with biocompatible focused ultrasound (FUS) by leveraging pressure-sensitive gas vesicles (GVs) as acousto-mechanical transducers. The power of this approach is illustrated through the mechanically triggered release of covalently bound fluorogenic and therapeutic cargo molecules from polymers containing a masked 2-furylcarbinol mechanophore. Molecular release occurs selectively in the presence of GVs upon exposure to FUS under physiological conditions. These results showcase the viability of this system for enabling remote control of specific mechanochemical reactions with spatiotemporal precision in biologically relevant settings and demonstrate the translational potential of polymer mechanochemistry.
转载本文请联系原作者获取授权,同时请注明本文来自诸平科学网博客。
链接地址:https://blog.sciencenet.cn/blog-212210-1423955.html
上一篇:烟酸过多会增加患心血管疾病的风险